Vaccine Cold Room Temperatures

Chiller Room (2°C to 8°C)
Chiller Room (2°C to 8°C)
Freezer Room (below -15°C)
Freezer Room (below -15°C)

Pharmaceutical Industry
Pharmaceutical Industry
Store vaccines at precise temperatures, ensuring their potency and safety before distribution to healthcare providers and clinics.

Healthcare and Hospitals
Healthcare and Hospitals
Biotechnology
Biotechnology

Non-Governmental Organizations
Non-Governmental Organizations
Comprehensive FAQ: Vaccine Cold Storage & Cold Chain Management
Q1: What is a Vaccine Cold Room, and how does it differ from a standard refrigerator?
A: A Vaccine Cold Room (VCR) is a walk-in refrigerated enclosure designed to store large volumes of vaccines at precise temperatures.
-
Capacity: Unlike a standard refrigerator, a VCR allows personnel to walk inside to organize, stack, and manage inventory on pallets or shelving units.
-
Stability: VCRs utilize heavy-duty insulation (Polyurethane panels) and industrial-grade refrigeration units, offering superior temperature stability compared to standalone fridges.
-
Redundancy: Standard fridges often lack backup systems. Vaccine cold rooms are almost always designed with 100% redundancy (dual refrigeration systems) to prevent failure.
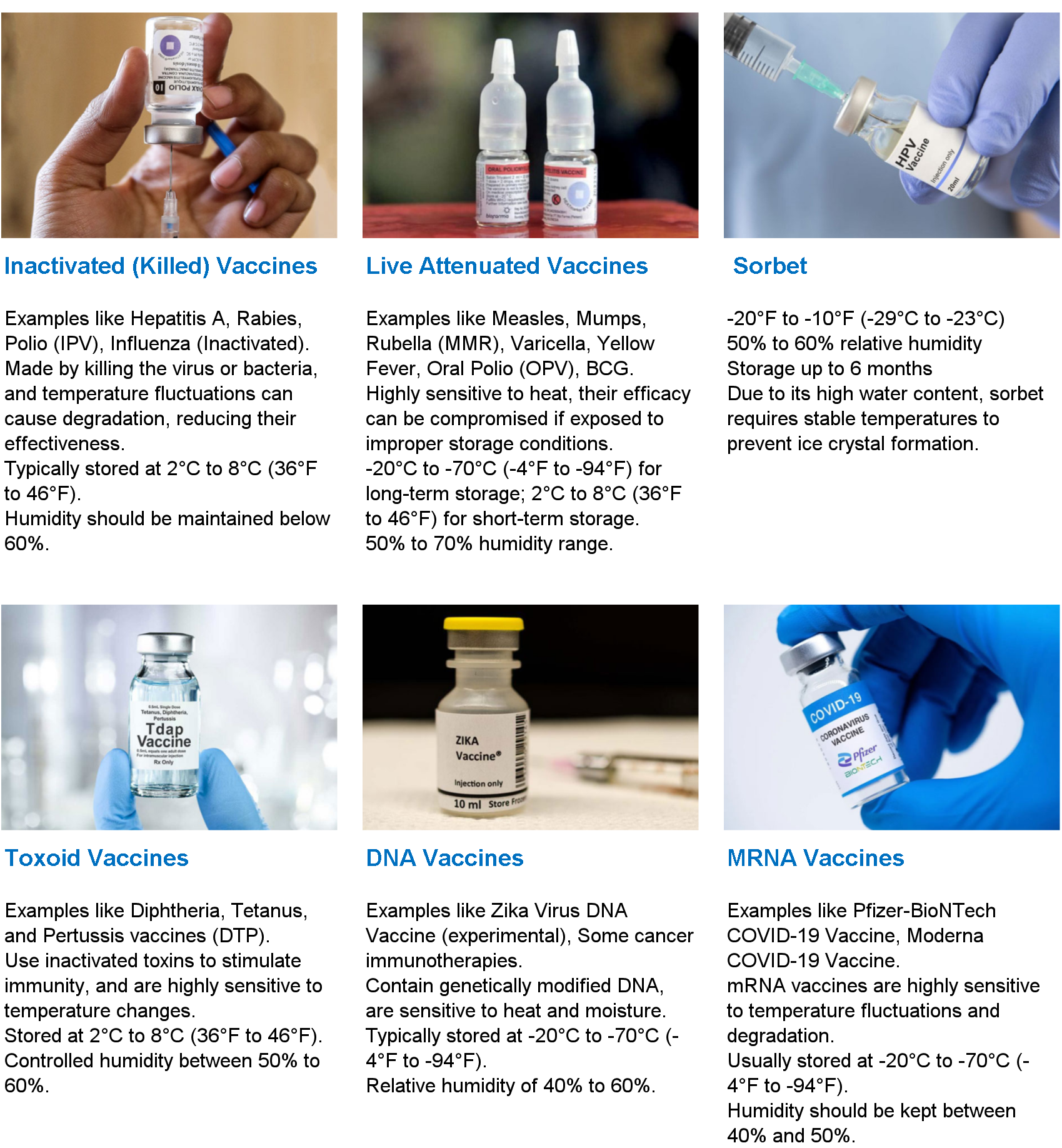
Q2: What are the standard temperature ranges for vaccine storage?
A: There are generally three categories, depending on the type of vaccine:
-
Cold Room (2°C to 8°C): This is the most common range for DTP, Tetanus, Hepatitis B, and Typhoid vaccines. The target set-point is usually 5°C.
-
Freezer Room (-15°C to -25°C): Required for vaccines like OPV (Oral Polio Vaccine) and some Measles vaccines.
-
Ultra-Low Temperature (ULT) Storage (-70°C to -80°C): Specifically required for certain mRNA vaccines (e.g., Pfizer-BioNTech COVID-19 vaccine). These are usually achieved using specialized ULT freezers housed within a cold room or an air-conditioned warehouse, rather than the room itself being cooled to -80°C.
Q3: What is the "Cold Chain," and why is the cold room considered a critical node?
A: The Cold Chain is the system of transporting and storing vaccines within a specific temperature range from the point of manufacture to the point of use.
The cold room acts as a critical node or a "hub" in this chain. If a cold room fails, thousands or millions of doses can be spoiled simultaneously (known as a "temperature excursion"). Once a vaccine loses potency due to heat exposure or accidental freezing, the damage is irreversible.
Q4: What materials are used for the insulation of a vaccine cold room?
A: The industry standard is Polyurethane (PU) Sandwich Panels.
-
Thickness: Typically 100mm for cold rooms (2~8°C) and 120mm-150mm for freezer rooms.
-
Density: High-density foam (40-42 kg/m³) is used to ensure thermal efficiency and structural integrity.
-
Cladding: The surface is usually Stainless Steel (304 grade) or Pre-painted Galvanized Iron (PPGI) with a food-safe, antibacterial coating. Stainless steel is preferred for medical hygiene as it is resistant to corrosion and harsh cleaning chemicals.
Q5: Why is a "Dual Refrigeration System" required for vaccine storage?
A: Medical guidelines (such as WHO PQS) strongly recommend or mandate a Run/Standby configuration.
-
Mechanism: The cold room is equipped with two independent refrigeration units.
-
Operation: Only one unit runs at a time. They alternate (e.g., every 12 hours) to equalize wear and tear.
-
Safety: If the primary unit fails, the standby unit automatically kicks in to maintain the temperature, ensuring zero downtime.
Q6: What is "Thermal Mapping," and is it necessary for the design phase?
A: Yes, it is necessary. Thermal mapping (or temperature mapping) is the process of analyzing the temperature distribution within the empty and loaded cold room.
-
Purpose: To identify "Hot Spots" (near doors or lights) and "Cold Spots" (near the evaporator airflow).
-
Outcome: The results determine where to place the monitoring sensors and where not to store sensitive vaccines.
Q7: What kind of monitoring system is required for a vaccine cold room?
A: A simple thermometer is insufficient. You require a Data Logging System (DLS) or a Building Management System (BMS). Key features include:
-
Continuous Recording: Records temperature every 5 to 10 minutes.
-
Remote Access: Allows users to view data via cloud/web dashboards.
-
Audit Trail: Data must be unalterable to comply with regulations (like FDA 21 CFR Part 11).
Q8: How do the alarm systems work?
A: The alarm system must be multi-modal. If the temperature deviates from the set range (e.g., rises above 7.5°C or falls below 2.5°C):
-
Local Alarm: A loud siren and strobe light outside the cold room.
-
Remote Alarm: SMS text messages, emails, or phone calls sent to designated personnel (Store Manager, Technician, Security).
-
Power Failure Alarm: An independent battery-operated alarm that notifies staff if the mains power is cut.
Q9: What is "Accidental Freezing," and why is it a risk in a cold room?
A: Accidental freezing occurs when vaccines (specifically freeze-sensitive ones like Hepatitis B) are placed too close to the evaporator unit where cold air blows directly.
-
Risk: Freezing destroys the adjuvant in the vaccine, rendering it useless and potentially causing adverse reactions in patients.
-
Prevention: Install wire mesh guards around the evaporator to prevent stacking boxes in the direct airflow path.
Q10: How should vaccines be arranged inside the cold room?
A: Proper arrangement is vital for air circulation:
-
Shelving: Use open-wire or perforated shelves (stainless steel).
-
Spacing: Leave a gap of at least 10cm between the boxes and the walls, and between the boxes and the floor (use pallets).
-
Airflow: Never block the evaporator fans.
-
Segregation: Quarantine areas (for expired or returned vaccines) must be clearly marked and physically separated from usable stock.
Q11: What is the FEFO principle?
A: FEFO stands for First Expired, First Out.
Unlike FIFO (First In, First Out), FEFO ensures that vaccines with the closest expiration dates are distributed first, minimizing wastage. The warehouse management system (WMS) or inventory cards should reflect this strategy.
Q12: How often should the temperature sensors be calibrated?
A: Sensors should be calibrated at least once a year (annually).
-
Standard: Calibration must be traceable to national or international standards (e.g., NIST).
-
3-Point Calibration: It is best practice to calibrate at three points (e.g., 0°C, 5°C, and 10°C) to ensure accuracy across the operating range.
Q13: What happens during a power outage? How long will the vaccines stay safe?
A: This depends on the "Hold-over Time."
-
Backup Generator: Facilities should have an automatic standby generator (ATS) that starts within 30-60 seconds of a power cut.
-
Hold-over Time: If the generator fails, a well-insulated PU cold room (100mm) kept closed can maintain temperature for 4 to 12 hours, depending on the ambient temperature and the volume of product inside (thermal mass).
-
Protocol: DO NOT open the door during a power outage unless absolutely necessary.
Q14: What is the "Emergency Contingency Plan"?
A: Every facility must have a written plan that answers:
-
Who is the primary contact person?
-
Where is the alternative storage location? (e.g., a nearby hospital or a refrigerated truck).
-
How to transport vaccines safely during an emergency?
-
Who repairs the refrigeration unit?
Q15: Routine maintenance checklist?
-
Daily: Check temperature logs, check for alarms, inspect door seals for gaps.
-
Monthly: Clean evaporator coils and condenser fins (dust reduces efficiency), check refrigerant levels.
-
Quarterly: Test the backup generator, test the remote alarm system (simulate a failure).
Q16: What is IQ, OQ, and PQ validation for cold rooms?
A: These are quality assurance protocols required by health authorities (like the FDA, WHO, or local equivalent):
-
IQ (Installation Qualification): Verifies that the cold room was built and installed according to the design specifications (e.g., checking panel thickness, motor voltage).
-
OQ (Operational Qualification): Verifies that the equipment operates correctly (e.g., testing that the alarm goes off at 8°C, testing the switch between primary and backup cooling units).
-
PQ (Performance Qualification): Verifies that the cold room maintains the temperature under real-world conditions (loaded with product, doors opening and closing) over a period (usually 24-72 hours).
Q17: Do I need a specific floor for a vaccine cold room?
A: Yes. The floor should be:
-
Insulated: To prevent cold loss into the ground and condensation/icing.
-
Non-slip: For worker safety.
-
Easy to clean: Usually aluminum checker plate or an epoxy resin finish (pharmaceutical grade) that does not harbor bacteria.
-
Excursion: Any temperature reading outside the recommended range.
-
Hermetic Compressor: A sealed compressor unit used in refrigeration.
-
Hydrofluorocarbons (HFCs): Common refrigerants (e.g., R404A, R134a) used in cooling systems. Check local environmental regulations regarding GWP (Global Warming Potential).
-
Data Logger: An electronic device that records temperature over time.
-
WHO PQS: World Health Organization Performance, Quality and Safety standards.
Tiempo de publicación:Sep-25-2020